We Speak Science!
We offer our customers a full suite of services that meet the needs of technical and scientific writing. Our reports are based on references recognized by the scientific community and in evidence-based medicine, including technical-scientific books, articles published in journals indexed by the WEB QUALIS system, electronic scientific bases of the Capes Journal Portal and official documents from Brazilian government institutions.We offer regulatory support for registration and post-registration of drugs, assisting our customers in defining strategies and preparing responses to requirements, in addition to providing technical defense with ANVISA.
The company stood out in monitoring relative bioavailability and bioequivalence studies, offering an excellent service that ensures complete safety in the processes monitored for our customers.
Our business approach values expertise, education and experience in the pharmaceutical research industry. We provide our customers with a wide variety of resources and skills to deliver projects as agreed.

SERVICES
MONITORING IN CLINICAL TRIALS
Phartrials monitors Clinical Research and Relative Bioavailability/Bioequivalence studies at an on-site study site, in compliance with Good Clinical Practice (ICH - GCP) regulations, with reporting to the Sponsor and Study Sites, including preventive and corrective actions.
We are enabled to perform in accordance with Good Clinical Practice (ICH – GCP) standards and in compliance with our own Quality System (SOPs) and guidelines, ensuring that the clinical study is conducted, recorded and reported in full compliance with the clinical protocol.
Clinical study monitoring aims to represent the sponsor at all stages of conducting a clinical study in the processes performed by the Study Site, from pre-study to post-study.
In addition to a highly qualified technical team to perform the monitoring service, Phartrials has as its differential a customized computerized system, used by the team via tablet, which allows real-time monitoring of the activities monitored during the stages of a clinical study. This is a differentiator that ensures that monitoring services are healthy and safe, in addition to optimizing monitoring processes.
Phartrials started with this service in 2020 and has performed more than 100 monitoring in Research Centers, with total satisfaction of Customers.
We are enabled to perform in accordance with Good Clinical Practice (ICH – GCP) standards and in compliance with our own Quality System (SOPs) and guidelines, ensuring that the clinical study is conducted, recorded and reported in full compliance with the clinical protocol.
Clinical study monitoring aims to represent the sponsor at all stages of conducting a clinical study in the processes performed by the Study Site, from pre-study to post-study.
In addition to a highly qualified technical team to perform the monitoring service, Phartrials has as its differential a customized computerized system, used by the team via tablet, which allows real-time monitoring of the activities monitored during the stages of a clinical study. This is a differentiator that ensures that monitoring services are healthy and safe, in addition to optimizing monitoring processes.
Phartrials started with this service in 2020 and has performed more than 100 monitoring in Research Centers, with total satisfaction of Customers.
SERVICES
MONITORING IN CLINICAL TRIALS
Phartrials monitors Clinical Research and Relative Bioavailability/Bioequivalence studies at an on-site study site, in compliance with Good Clinical Practice (ICH - GCP) regulations, with reporting to the Sponsor and Study Sites, including preventive and corrective actions.
We are enabled to perform in accordance with Good Clinical Practice (ICH – GCP) standards and in compliance with our own Quality System (SOPs) and guidelines, ensuring that the clinical study is conducted, recorded and reported in full compliance with the clinical protocol.
Clinical study monitoring aims to represent the sponsor at all stages of conducting a clinical study in the processes performed by the Study Site, from pre-study to post-study.
In addition to a highly qualified technical team to perform the monitoring service, Phartrials has as its differential a customized computerized system, used by the team via tablet, which allows real-time monitoring of the activities monitored during the stages of a clinical study. This is a differentiator that ensures that monitoring services are healthy and safe, in addition to optimizing monitoring processes.
Phartrials started with this service in 2020 and has performed more than 100 monitoring in Research Centers, with total satisfaction of Customers.
We are enabled to perform in accordance with Good Clinical Practice (ICH – GCP) standards and in compliance with our own Quality System (SOPs) and guidelines, ensuring that the clinical study is conducted, recorded and reported in full compliance with the clinical protocol.
Clinical study monitoring aims to represent the sponsor at all stages of conducting a clinical study in the processes performed by the Study Site, from pre-study to post-study.
In addition to a highly qualified technical team to perform the monitoring service, Phartrials has as its differential a customized computerized system, used by the team via tablet, which allows real-time monitoring of the activities monitored during the stages of a clinical study. This is a differentiator that ensures that monitoring services are healthy and safe, in addition to optimizing monitoring processes.
Phartrials started with this service in 2020 and has performed more than 100 monitoring in Research Centers, with total satisfaction of Customers.
PREPARATION OF TECHNICAL-SCIENTIFIC DOCUMENTATION AND/OR EVALUATION TO COMPLY WITH REGULATORY STANDARDS
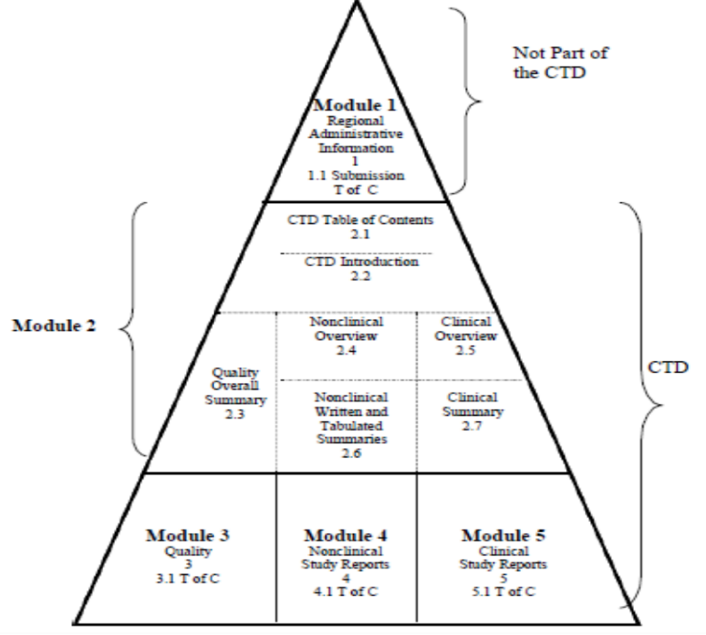
Technical Documentation in CTD format (according to Guide 24/2019), common format for the preparation of an application for registration and post-registration of well-structured drug to be submitted to ANVISA:
- Administrative Information (module 1) – Specific to ANVISA;
- CTD index (2.1);
- CTD Introduction (2.2.);
- Non-clinical Modules (2.4, 2.6 and 4):
Bibliographic Survey for the composition of the Technical Documentation
Non-clinical Overview
Tabulated and Written Nonclinical Summaries (Pharmacology, Pharmacokinetics, and Toxicology)
Nonclinical Study Reports
- Clinical Modules (2.5, 2.7 and 5):
Systematic Review of the Literature of Clinical Studies
Clinical Overview
Clinical Summaries (Biopharmaceutic Studies, Analytical Methods, Clinical Pharmacology, Efficacy and Safety)
Clinical Trial Reports
- Administrative Information (module 1) – Specific to ANVISA;
- CTD index (2.1);
- CTD Introduction (2.2.);
- Non-clinical Modules (2.4, 2.6 and 4):
Bibliographic Survey for the composition of the Technical Documentation
Non-clinical Overview
Tabulated and Written Nonclinical Summaries (Pharmacology, Pharmacokinetics, and Toxicology)
Nonclinical Study Reports
- Clinical Modules (2.5, 2.7 and 5):
Systematic Review of the Literature of Clinical Studies
Clinical Overview
Clinical Summaries (Biopharmaceutic Studies, Analytical Methods, Clinical Pharmacology, Efficacy and Safety)
Clinical Trial Reports
Drug Clinical Development Dossier (DCDD), compiled from documents to be submitted to ANVISA for the purpose of evaluating the stages inherent to the development of an experimental drug in order to obtain information to support the registration or post-registration changes of said product.
Investigator’s Brochure, a document that contains the compilation of the nonclinical and clinical data of an investigational medicinal product that are relevant to the study in humans, with the purpose of providing investigators and others involved in the conduct of the clinical trial with information regarding the dose, dosing regimen, methods of administration and safety monitoring procedures.
Pharmaceutical Bioequivalence study protocols, document that describes the objectives, design, methodology, statistical considerations, and organization of the trial. It also provides the context and rationale for the clinical trial.
Relative bioavailability/bioequivalence studies, evaluation of clinical, bioanalytical, and statistical data and information (reporting) to identify regulatory adequacy needs.
Technical-Scientific Reports:
- Efficacy and safety – specific drugs;
- Linear pharmacokinetics for biowaiver.
Making package inserts, with information on prescribing, preparation, administration, warning and other guidelines for safe use and effective treatment.
Investigator’s Brochure, a document that contains the compilation of the nonclinical and clinical data of an investigational medicinal product that are relevant to the study in humans, with the purpose of providing investigators and others involved in the conduct of the clinical trial with information regarding the dose, dosing regimen, methods of administration and safety monitoring procedures.
Pharmaceutical Bioequivalence study protocols, document that describes the objectives, design, methodology, statistical considerations, and organization of the trial. It also provides the context and rationale for the clinical trial.
Relative bioavailability/bioequivalence studies, evaluation of clinical, bioanalytical, and statistical data and information (reporting) to identify regulatory adequacy needs.
Technical-Scientific Reports:
- Efficacy and safety – specific drugs;
- Linear pharmacokinetics for biowaiver.
Making package inserts, with information on prescribing, preparation, administration, warning and other guidelines for safe use and effective treatment.
PREPARATION OF TECHNICAL-SCIENTIFIC DOCUMENTATION AND/OR EVALUATION TO COMPLY WITH REGULATORY STANDARDS
Technical Documentation in CTD format (according to Guide 24/2019), common format for the preparation of an application for registration and post-registration of well-structured drug to be submitted to ANVISA:
- Administrative Information (module 1) – Specific to ANVISA;
- CTD index (2.1);
- CTD Introduction (2.2.);
- Non-clinical Modules (2.4, 2.6 and 4):
Bibliographic Survey for the composition of the Technical Documentation
Non-clinical Overview
Tabulated and Written Nonclinical Summaries (Pharmacology, Pharmacokinetics, and Toxicology)
Nonclinical Study Reports
- Clinical Modules (2.5, 2.7 and 5):
Systematic Review of the Literature of Clinical Studies
Clinical Overview
Clinical Summaries (Biopharmaceutic Studies, Analytical Methods, Clinical Pharmacology, Efficacy and Safety)
Clinical Trial Reports
- Administrative Information (module 1) – Specific to ANVISA;
- CTD index (2.1);
- CTD Introduction (2.2.);
- Non-clinical Modules (2.4, 2.6 and 4):
Bibliographic Survey for the composition of the Technical Documentation
Non-clinical Overview
Tabulated and Written Nonclinical Summaries (Pharmacology, Pharmacokinetics, and Toxicology)
Nonclinical Study Reports
- Clinical Modules (2.5, 2.7 and 5):
Systematic Review of the Literature of Clinical Studies
Clinical Overview
Clinical Summaries (Biopharmaceutic Studies, Analytical Methods, Clinical Pharmacology, Efficacy and Safety)
Clinical Trial Reports
Drug Clinical Development Dossier (DCDD), compiled from documents to be submitted to ANVISA for the purpose of evaluating the stages inherent to the development of an experimental drug in order to obtain information to support the registration or post-registration changes of said product.
Investigator’s Brochure, a document that contains the compilation of the nonclinical and clinical data of an investigational medicinal product that are relevant to the study in humans, with the purpose of providing investigators and others involved in the conduct of the clinical trial with information regarding the dose, dosing regimen, methods of administration and safety monitoring procedures.
Pharmaceutical Bioequivalence study protocols, document that describes the objectives, design, methodology, statistical considerations, and organization of the trial. It also provides the context and rationale for the clinical trial.
Relative bioavailability/bioequivalence studies, evaluation of clinical, bioanalytical, and statistical data and information (reporting) to identify regulatory adequacy needs.
Technical-Scientific Reports:
- Efficacy and safety – specific drugs;
- Linear pharmacokinetics for biowaiver.
Making package inserts, with information on prescribing, preparation, administration, warning and other guidelines for safe use and effective treatment.
Investigator’s Brochure, a document that contains the compilation of the nonclinical and clinical data of an investigational medicinal product that are relevant to the study in humans, with the purpose of providing investigators and others involved in the conduct of the clinical trial with information regarding the dose, dosing regimen, methods of administration and safety monitoring procedures.
Pharmaceutical Bioequivalence study protocols, document that describes the objectives, design, methodology, statistical considerations, and organization of the trial. It also provides the context and rationale for the clinical trial.
Relative bioavailability/bioequivalence studies, evaluation of clinical, bioanalytical, and statistical data and information (reporting) to identify regulatory adequacy needs.
Technical-Scientific Reports:
- Efficacy and safety – specific drugs;
- Linear pharmacokinetics for biowaiver.
Making package inserts, with information on prescribing, preparation, administration, warning and other guidelines for safe use and effective treatment.
AUDIT FOR QUALIFICATION OF PHARMACEUTICAL BIOEQUIVALENCE CENTER
Ensure, through auditing the Research Center, compliance with processes and Good Clinical Practices for carrying out Pharmaceutical Bioequivalence studies to qualify it as a supplier.
AUDIT FOR QUALIFICATION OF PHARMACEUTICAL BIOEQUIVALENCE CENTER
Ensure, through auditing the Research Center, compliance with processes and Good Clinical Practices for carrying out Pharmaceutical Bioequivalence studies to qualify it as a supplier.
AUDIT FOR QUALIFICATION OF PHARMACEUTICAL EQUIVALENCE CENTER (EQFAR)
Ensure compliance of the Pharmaceutical Equivalence Center's processes and Good Laboratory Practices in Pharmaceutical Equivalence tests for Drugs to qualify it as a supplier.
AUDIT FOR QUALIFICATION OF PHARMACEUTICAL EQUIVALENCE CENTER (EQFAR)
Ensure compliance of the Pharmaceutical Equivalence Center's processes and Good Laboratory Practices in Pharmaceutical Equivalence tests for Drugs to qualify it as a supplier.

Avenida Anhanguera, qd. 38 lt. 96, Nº 4803, Sala 1404, Ed. Rita de Albuquerque, Setor Central, Goiânia-GO, CEP: 740430-11
COMPANY - WHO WE ARE?
QUALITY POLICY
MISSION
VISION
VALUES
SOCIAL AND ENVIRONMENTAL RESPONSABILITY POLICY
TEAM
QUALITY POLICY
MISSION
VISION
VALUES
SOCIAL AND ENVIRONMENTAL RESPONSABILITY POLICY
TEAM
OUR SERVICES
WE SPEAK SCIENCE!
MONITORING IN CLINICAL TRIALS
PREPARATION OF THECNICAL-SCIENTIFIC DOCUMENTATION AND/OR EVOLUATION TO COMPLY WHITH REGULATORY STANDARTS
AUDIT FOR QUALIFICATION OF PHARMACEUTICAL BIOEQUIVALENCE CENTER
WE SPEAK SCIENCE!
MONITORING IN CLINICAL TRIALS
PREPARATION OF THECNICAL-SCIENTIFIC DOCUMENTATION AND/OR EVOLUATION TO COMPLY WHITH REGULATORY STANDARTS
AUDIT FOR QUALIFICATION OF PHARMACEUTICAL BIOEQUIVALENCE CENTER
AUDIT FOR QUALIFICATION OF PHARMACEUTICAL EQUIVALENCE CENTER (EQFAR)
AUDIT IN THE QUALITY MANAGEMENT SYSTEM
CONTACT
AUDIT IN THE QUALITY MANAGEMENT SYSTEM
CONTACT



